November 11 RxAdvocate September, 2023 — Newsletter
September Stories:
- FDA Approves Vyvanse Generics
- BCBS/California Drops CVS
- FDA Approves Abrysvo
- Community Involvement
FDA Approves Vyvanse Generics Amid ADHD Medication Shortage
The FDA has approved generic versions of Vyvanse, a popular medication used to treat ADHD and binge eating disorder. Now, more than a dozen companies will be able to manufacture more affordable generic options. These generic medicines are designed to work in the same way and provide the same benefit as the brand.
Though it is still unclear when these generics will hit pharmacies, the approval offers hope for many individuals who have been facing numerous ADHD medication shortages. Expect to see these medications hit PBM formularies in about 60 days.
Blue Cross Blue Shield of California Drops CVS Caremark
Blue Shield of California, a nonprofit health plan with nearly 5 million members, has announced a radical plan to jettison its legacy pharmacy benefit manager. Here’s what their decision could mean for the future of healthcare in America:
CVS Caremark just lost a major customer – sort of. In a move that The Wall Street Journal described as “ripping up the playbook,” Blue Shield of California has limited their involvement with Caremark to the handling of specialty drug services only, devolving all other pharmacy plan responsibilities to a suite of alternative vendors such as Amazon Pharmacy and Mark Cuban’s Cost Plus Drug Company.
Pharmacy Benefit Managers (PBMs) such as Caremark have been increasingly scrutinized for their opaque and complex role in prescription drug spending in America, which saw a 200-fold increase from 1967 to 2021. It is important for industry observers to understand the possible ramifications of this decision and its potential impact on the employer pharmacy benefit landscape. Click the link below to view five key insights on Blue Shield’s bold play:
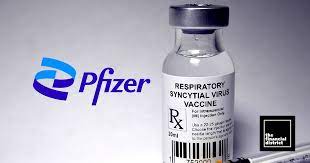
FDA Approves ABRYSVO for Prevention of RSV in Infants for Pregnant Individuals
FDA Approves ABRYSVO for Prevention of RSV in Infants for Pregnant Individuals

Pfizer Inc. announced that the U.S. Food and Drug Administration has approved ABRYSVO, the first vaccine for pregnant women to protect their newborns against respiratory syncytial virus (RSV). It has been approved for women to receive the vaccine who have been pregnant for 32 to 36 weeks.
According to the CDC, RSV is the leading cause of hospitalization of infants. Most individuals can be expected to be infected with RSV by the time they reach two years of age. While RSV most often causes cold-like symptoms in infants and young children, it can also lead to serious LRTD such as pneumonia and bronchiolitis (swelling of the small airway passages in the lungs).
Among 3,500 pregnant individuals who received ABRYSVO, compared to 3,500 pregnant individuals who received placebo, ABRYSVO reduced the risk of severe LRTD (Lower Respiratory tract Disease) by 81.8% within 90 days after birth, and 69.4% within 180 days after birth. The most commonly reported side effects by pregnant individuals who received Pfizer’s ABRYSVO were pain at the injection site, headache, muscle pain and nausea. For more details on ABRYSVO
Back to School Season!
Back to School Season!
RxConnection wishes you and your family a very happy back-to-school season!



Contact us
Visit us on the web at www.rxconnectionllc.com.
If you would like additional information on our services please contact:
Orlando Neal — Principal
Orlando.Neal@rxconnectionllc.com
Orlando.Neal@rxconnectionllc.com





